Capturing and controlling the fleeting dance of electrons as they rearrange during a chemical reaction has been a long-standing challenge in science for several decades. Since electrons are much lighter than atoms, they can respond almost instantaneously – on time scales of hundreds of attoseconds, where an attosecond is 10-18 s.
Fortunately, over the last decade scientists have created attosecond x-ray strobe lights that are fast enough to freeze the motion of electrons. However, simply illuminating a molecule with an attosecond flash of x-ray light would not work because one law of quantum mechanics dictates that an attosecond burst of light must span a broad energy range. Thus, scientists could not tell how the energy of an electron changes during a chemical reaction; nor could they use laser light to steer the outcome of a chemical reaction.
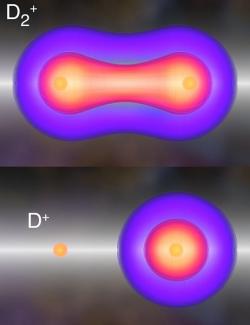
Fortunately, the Kapteyn/Murnane group has learned how to make a multicolor light field consisting of a series of finely tuned attosecond bursts of high-energy ultraviolet (UV) laser-like light of carefully selected wavelengths. Using this optimized light field, they were able to coax an electron to hop from one energy level to another on attosecond time scales. The researchers used this ability to simultaneously control both the electrons and atoms in a deuterium (D2) molecule.
This exquisite control allowed them to dictate the exact pathway by which the molecule loses an electron (ionizes), regulate the way the molecule vibrates, and even manipulate the way in which the molecule falls apart. Figuring out how to use quantum physics to control chemical reactions even in a simple molecule like D2 was both daunting and remarkable.
The researchers responsible for this important breakthrough include former research associate Predrag Ranitovic, graduate student Craig Hogle, former undergraduate Leigh Martin, Fellows Margaret Murnane and Henry Kapteyn, as well as colleagues from the University of Tsukuba (Japan), Universidad Autónoma de Madrid (Spain), and Instituto Madrileño de Estudios Avanzados en Nanociencia (Spain).
The Kapteyn/Murnane group expects this breakthrough to lead to new and better ideas for using ultrafast lasers and x-ray pulses to control chemical reactions.—Margaret Murnane and Julie Phillips